ocrevus start form pdf
Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. OCREVUS is aCD20-directed cytolytic antibody indicated for the treatment of.

Recent Advances In Nanomedicines For Multiple Sclerosis Therapy Acs Applied Bio Materials
M F Patient Address.

. Ocrevus Order FormPlease fax form to. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and. OCREVUS is a prescription medicine used to treat.
Medication orders can be placed with Accredo via E-prescribe - Accredo 1640 Century Center Pkwy Memphis TN. Ocrevus ocrelizumab 02-micron filter must be used during infusion Initial dosing. It is a one-time registration completed by the.
_____ Current Patient New Patient Need by date. It must be completed by the provider. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary.
Ocrevus 600mg500ml IV every 6 months 24 weeks. The form includes patient insurance and prescription information. Relapsing forms of multiple sclerosis MStoincludeclinically isolated syndrome relapsing-remitting disease.
650 877-1111 Call Visit Online Fax Genentech. Duration should be at least 35 hrs. OCREVUS is a prescription medicine used to treat.
This form is used to initiate the EFT registration process when the practice chooses not to use check reimbursements. 2 DOSAGE AND ADMINISTRATION 21 Assessments Prior to. Infuse 300mg IV in 250ml NS over a minimum of 25 hours on day 0 and 14.
Date of birth Prescribers first name. OCREVUS is indicated for the treatment of. Start at 40mlhr increasing by 40mlhr every 30 min to a max rate of 200mlhr.
OCREVUS is indicated for the treatment of adult patients with relapsing or primary progressive forms of multiple sclerosis. OCREVUS START FORM Y Medical Associates Fax Referral To. Prescription Enrollment Form.
OCREVUS was not associated with. If no Please provide cl inical support for continued use of Ocrevus. The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions.
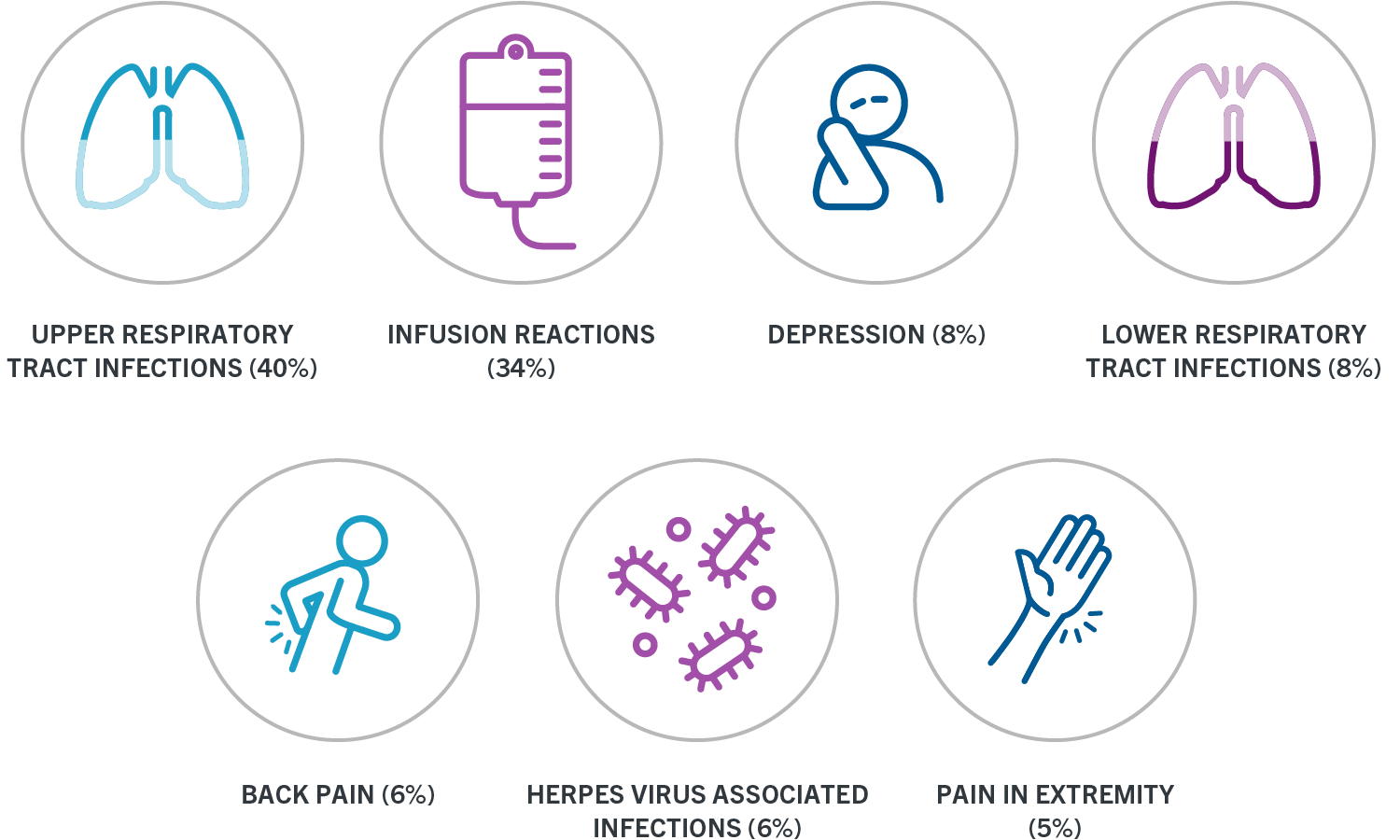
405- 726-9849 Patient Information Patient Name. Ocrevus ocrelizumab Fax completed form to 8883021028. OCREVUS increased the risk for upper respiratory tract infections lower respiratory tract infections skin infections and herpes-related infections.

Safety Of Ocrelizumab In Patients With Relapsing And Primary Progressive Multiple Sclerosis Neurology

Ocrevus Start Form Pdf Fill Online Printable Fillable Blank Pdffiller

Free Humana Prior Rx Authorization Form Pdf Eforms
Ocrelizumab A Review In Multiple Sclerosis Springerlink

Safety Of Ocrelizumab In Patients With Relapsing And Primary Progressive Multiple Sclerosis Neurology
Ocrevus Side Effects What They Are And How To Manage Them

Ocrevus Ocrelizumab Multiple Sclerosis Ms Treatment

Ocrevus Side Effects Cost Uses And More

I M About To Start Ocrevus Multiple Sclerosis News Today Forums

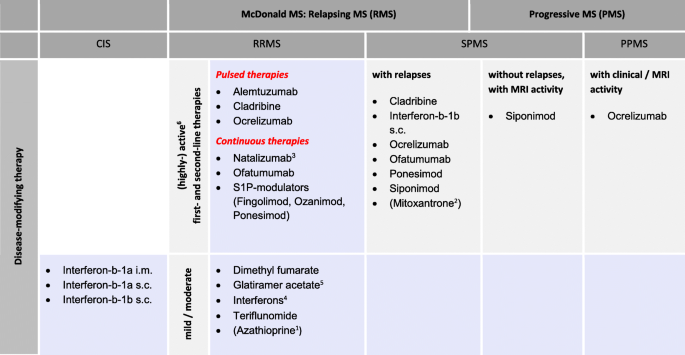
Controversy On The Treatment Of Multiple Sclerosis And Related Disorders Positional Statement Of The Expert Panel In Charge Of The 2021 Dgn Guideline On Diagnosis And Treatment Of Multiple Sclerosis Neuromyelitis Optica

Fillable Online Infusion Checklist For Ocrevus In Relapsing Or Fax Email Print Pdffiller
Multiple Sclerosis Therapy Consensus Group Mstcg Answers To The Discussion Questions Neurological Research And Practice Full Text

Ocrevus Ocrelizumab Multiple Sclerosis Ms Treatment

Thank You Ocrevus Roche Ups Guidance As Ms Drug Steams Past Blockbuster Mark Fierce Pharma

Roche Playing Defense Against Novartis Kesimpta Touts Ocrevus Benefits In Treating Ms Early Fierce Pharma

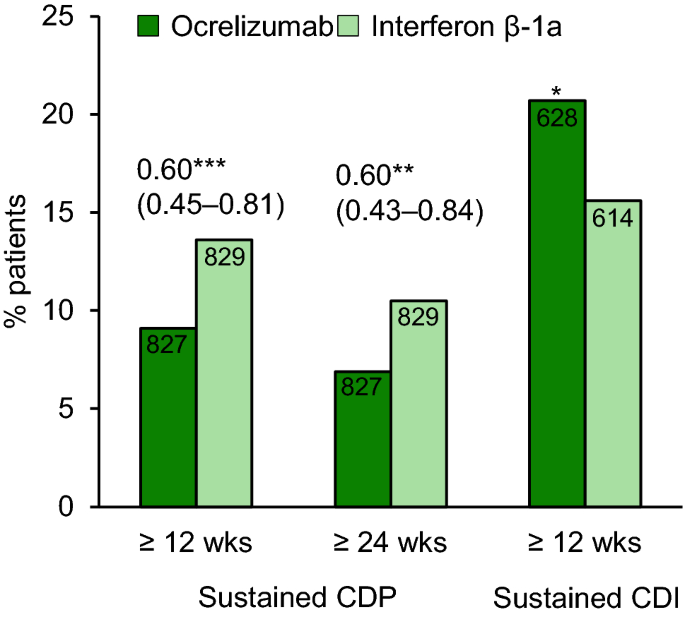
Pdf Onset Of Clinical And Mri Efficacy Of Ocrelizumab In Relapsing Multiple Sclerosis

Effect Of Ocrelizumab On Vaccine Responses In Patients With Multiple Sclerosis Neurology

Fillable Online My Relapsing Ms Brochure Ocrevus Fax Email Print Pdffiller